Counteraction of innate sensing and retroviral restriction by patient-derived HIV‑1 Vpr

Project Member

Prof. Dr. Michael Schindler
Head of Department Molecular Virology
Institute for Medical Virology
and Epidemiology of Viral Diseases
University Hospital Tübingen
Elfriede-Aulhorn-Straße 6
72076 Tübingen
Germany

Anthea Darius,
PhD Student
Project Summary
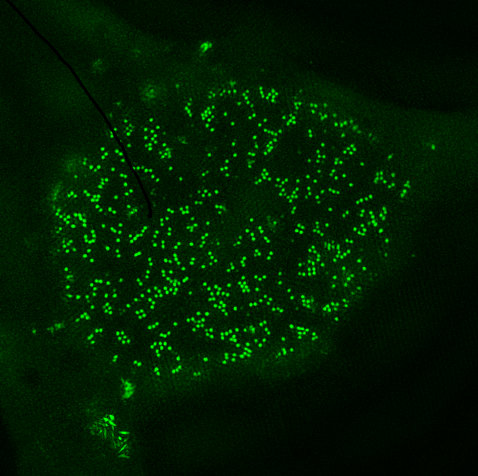
Vpr is the HIV‑1 accessory protein with the highest abundance in the virion and is delivered directly into the cytoplasm of the infected cell before de novo production of viral proteins. Furthermore, it has various described but still enigmatic functions related to the enhancement of HIV‑1 integration and increased viral gene expression and new results support a role for Vpr in evasion of HIV‑1 immune sensing. We here hypothesize that counteraction of innate sensing and retroviral restriction by HIV‑1 Vpr is a crucial determinant of high viral loads and diseases progression in vivo. We will challenge this hypothesis by the comprehensive functional analyses of Vpr proteins isolated from a cohort of therapy naïve HIV‑1 infected individuals differing in viral loads and CD4+ T cell counts. In detail, we will investigate the ability of primary Vpr alleles to activate the SLX4 complex, induce G2/M arrest, associate with p21, counteract SamHD1, promote HIV‑1 infection of myeloid cells and to block interferon induction. Recently, these mainly novel Vpr functions were proposed as important for Vprs putative role in antagonism of HIV‑1 immune sensing and expression of antiviral restriction factors. It is unknown if there is a mechanistic link between these functions, if they synergize or whether these mechanisms operate independently to subvert the innate immune response against HIV‑1. Moreover, Vpr domains involved in mediating the various activities are elusive. Altogether, our comprehensive analyses of primary patient derived Vpr alleles supported by a mutagenesis approach will give novel mechanistic insights on the subversion of innate immune sensing and retroviral restriction by Vpr. Furthermore, we will reveal the relative importance of the investigated Vpr functions for HIV‑1 control in vivo.