The Role of TREX1 for Innate Sensing of Human Endogenous Retroviruses


Project Member

Dr. Alessia Ruggieri
Project Leader
Dr. Alessia Ruggieri
Phone: +49 6221 56 – 7761
Alessia.Ruggieri@med.uni-heidelberg.de
Department of Infectious Diseases
Molecular Virology
University Hospital Heidelberg
Im Neuenheimer Feld 344
69120 Heidelberg
Germany

Prof. Dr. Oliver Till Fackler
Project Leader
Prof. Dr. Oliver Till Fackler
Phone: +49 6221 56 – 1322
Oliver.Fackler@med.uni-heidelberg.de
Department of Infectious Diseases
Integrative Virology
University Hospital Heidelberg
Im Neuenheimer Feld 344
69120 Heidelberg
Germany

Julia Welsch,
PhD Student
Project Summary
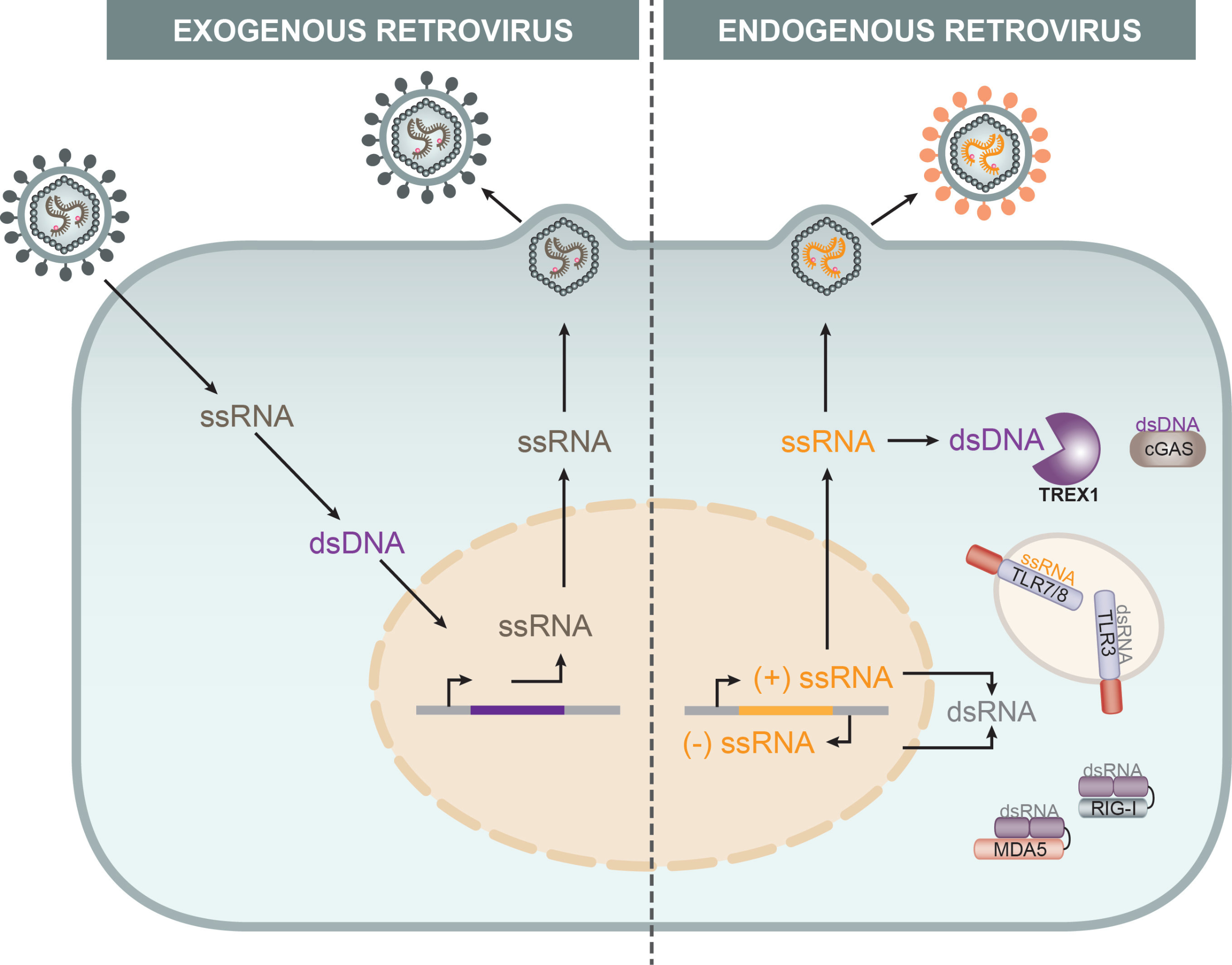
HIV‑1 reverse transcription (RT) products are predominantly sensed in most cell types by the cyclic GMP-AMP synthase cGAS-STING pathway. The host cell cytoplasmic exonuclease TREX1 was described to metabolize exogenous retroviral RT products in the cytoplasm of mammalian cells and studies in trex1 knock-out mice suggested that TREX1 has an important role in the elimination of ERV RT products as well as of cytoplasmic DNA products resulting from DNA damage. Based on these results reported in the literature, a central hypothesis of this project was that TREX1 plays a central role in controlling innate (auto) immune responses by lowering the amounts of retroviral and cellular cytoplasmic DNA to avoid innate immune sensing. Work in the first funding period established innate immune sensing competent and –incompetent cell systems with or without TREX1 expression for the analysis of TREX1 function in response to infection with exogenous retrovirus or upon induction of expression of endogenous retroviruses or DNA damage. In parallel, assays were established for the quantification of enzymatic activities of TREX1 in vitro and their immunological consequences in cells. Finally, the analysis of TREX1 expression in human cells yielded an unexpectedly complex pattern of cell-type and stimulation-dependent expression of various TREX1 isoforms that differ in their substrate specificity and revealed that TREX1 can be subject to proteolytic cleavage. Work in the second funding period will build on these tools and findings to achieve a comprehensive understanding of the role of diverse TREX1 isoforms and processing products in human cells in the context of retrovirus infection and DNA damage. Since TREX1 is a transmembrane protein localized at the endoplasmic reticulum as well as in the nucleus, another central question is how and where the exonuclease has access to retroviral RT products. To address these questions, we will conduct (i) a comprehensive functional characterization of TREX1 isoforms and cleavage products and (ii) dissect the immunological consequences of the metabolism of cytoplasmic DNA. These analyses will systematically compare exogenous infections with HIV‑1 with the induction of ERV expression or DNA damage. Together, we aim at providing novel tempo-spatial and mechanistic insight in the interplay between TREX1 and retroviral replication.