Antagonism of host cell restriction and sensing by HIV‑1 Nef


Project Member

Prof. Dr. Oliver Till Fackler
Project Leader
Prof. Dr. Oliver Till Fackler
Phone: +49 6221 56 – 1322
Oliver.Fackler@med.uni-heidelberg.de
Department of Infectious Diseases
Integrative Virology
University Hospital Heidelberg
Im Neuenheimer Feld 344
69120 Heidelberg
Germany

Leon Daniau,
PhD Student
Project Summary
The putative Serine Incorporators SERINC3 (S3) and SERINC5 (S5) were recently described as potent inhibitors of the infectivity of HIV particles that are efficiently counteracted by HIV‑1 Nef. The current mechanistic model of S3/5 as anti-retroviral restriction factors predicts that virion incorporation of S3/5 is a prerequisite for restriction and that Nef reroutes the restriction factors away from the plasma membrane of virus producing cells to exclude them from virions and thus maintain their infectivity. Our preliminary results obtained in preparation of this proposal defined a complex set of molecular determinants in Nef that govern S5 antagonism and suggest that intracellular rerouting of the restriction factor may be dispensable for antagonism.
Building on these initial findings, we now aim at defining molecular principles of the S3/5‑mediated restriction of HIV‑1 particle infectivity and Nef antagonism thereof and at assessing in which physiologically relevant primary cells the SERINC restriction is at work. In addition to structure-function analyses in cell lines and primary cells, this will include the analysis of virion morphology and lipid composition as well as the identification of involved host cell machinery. Our preliminary evidence also suggests that S5 sensitizes HIV particles to innate immune recognition and that Nef also antagonizes this activity. A second line of investigation of this project will therefore address the immunological consequences of the SERINC restriction.
These analyses will focus on defining the specificity and antiviral potency of this response, identifying viral and host cell components eliciting this response, and dissecting the mechanisms by which Nef antagonizes this effect. Together, these studies will yield insight into the molecular mechanisms of SERINC restriction and Nef antagonism as well as the patho-physiological consequences of the SERINC restriction.
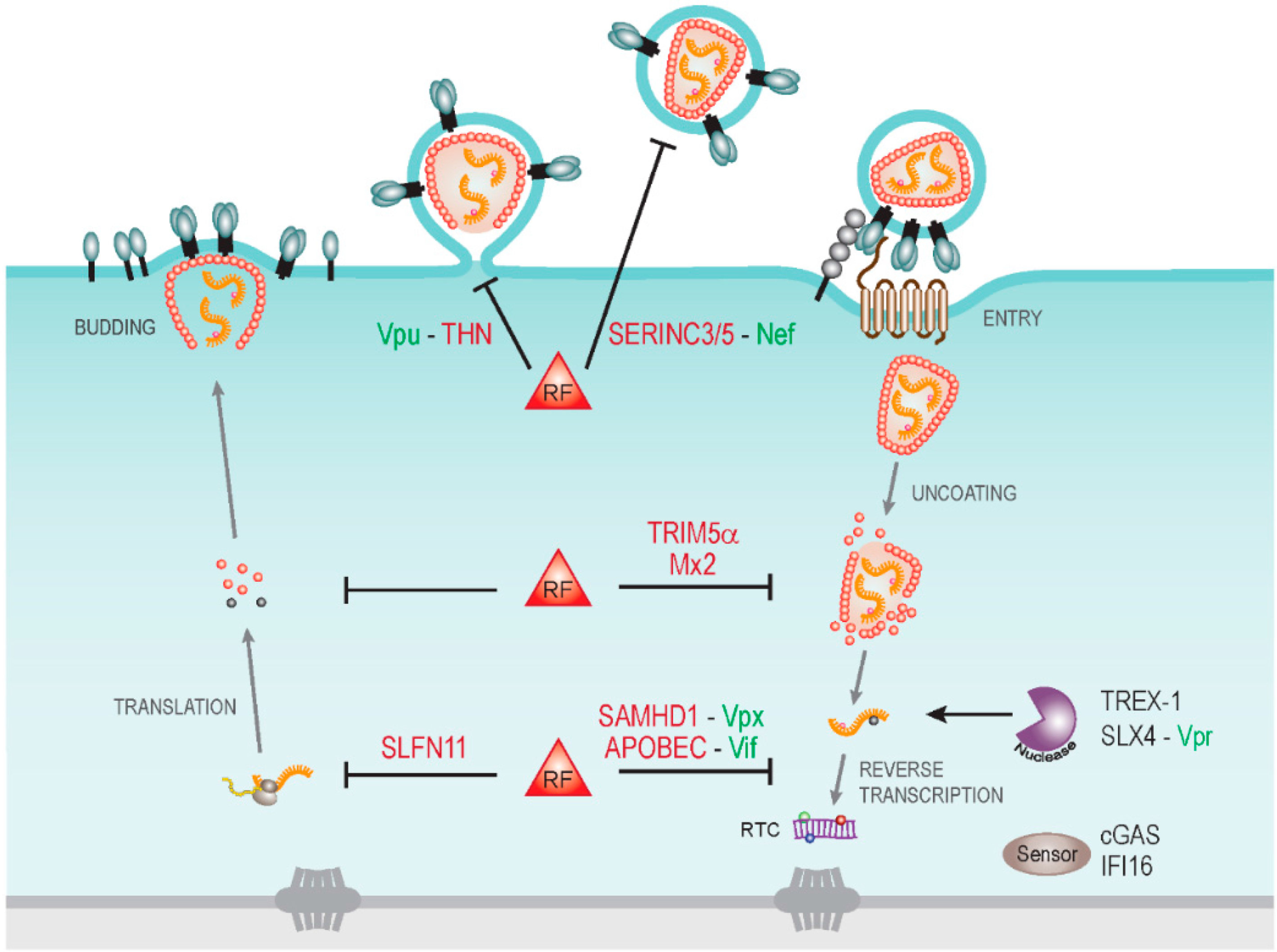
Fig. 1: Cytoplasmic host cell restrictions to human immunodeficiency virus types 1 (HIV‑1) infection and virally encoded antagonists. Schematic depiction of the HIV‑1 life cycle in the cytoplasm of a target cell with some restriction factors (RF) and their viral antagonists indicated. Early post entry steps of HIV‑1 replication are particularly targeted by host cell restriction factors including TRIM5α and Mx2 that recognize viral cores and may affect their stability. Uncoating of viral capsids renders viral RNA genomes accessible to host cell nucleases such as TREX1 that reduce innate immune recognition by the host cell and thus benefit HIV replication. Such a strategy may be exploited by the HIV‑1 Vpr protein that activates the SLX4 endonuclease complex. Reverse transcription of viral RNA genomes into DNA is targeted by the cytidine deaminase activity of the apolipoprotein B mRNA editing enzyme, catalytic polypeptide-like (APOBEC) proteins and the triphosphohydrolase SAMHD1, which are antagonized by the viral proteins Vif and Vpx (Vpx is only encoded bythe human immunodeficiency virus type 2 (HIV‑2) and the simian immunodeficiency virus (SIV) and is lacking in HIV‑1). Reverse transcription products are recognized by cytoplasmic DNA sensors such as cGAS and Ifi-16 to trigger innate immune responses. During virus production, translation of viral mRNA can be restricted by Schlafen11 (SLFN11). At the late stages of particle production, viral progeny is trapped at the cell surface by tetherin (THN), a restriction antagonized by Vpu. The infectivity of released particles can be significantly compromised by the newly described restriction factors serine incorporator 3 and 5 (SERINC3/5) and their antiviral activity is antagonized by Nef.
From: Fackler, O.T. (2015). Spotlight on HIV‑1 Nef: SERINC3 and SERINC5 identified as restriction factors antagonized by the pathogenesis factor. Viruses, 7: 6730 — 6738.